Preparation System
BRIEF INTRODUCTION
Standalone Positioning
Independently completes the compounding (liquid preparation) process, fully meeting GMP requirements for standalone production.
Applicable Dosage Forms & Specifications
Dosage Forms: Lyophilized powder for injection / aqueous injection vials, infusion bottles, prefilled syringes, liposomes, ADCs, vaccines, etc.
Batch Volume: 50 – 10,000 L
Design Capacity: ≤10,000 L per batch, with continuous buffer discharge up to 2,000 L/h
Process Flow
Manual / Automatic Charging → Integrated Process of This Unit: Vacuum Conveying → Raw & Auxiliary Material Feeding → Weighing → Dissolution → Mixing → Temperature Adjustment → Sterilizing Filtration → Dynamic Buffering → Aseptic Transfer → Aseptic Transfer to Downstream Filling Machines
Key Performance Parameters
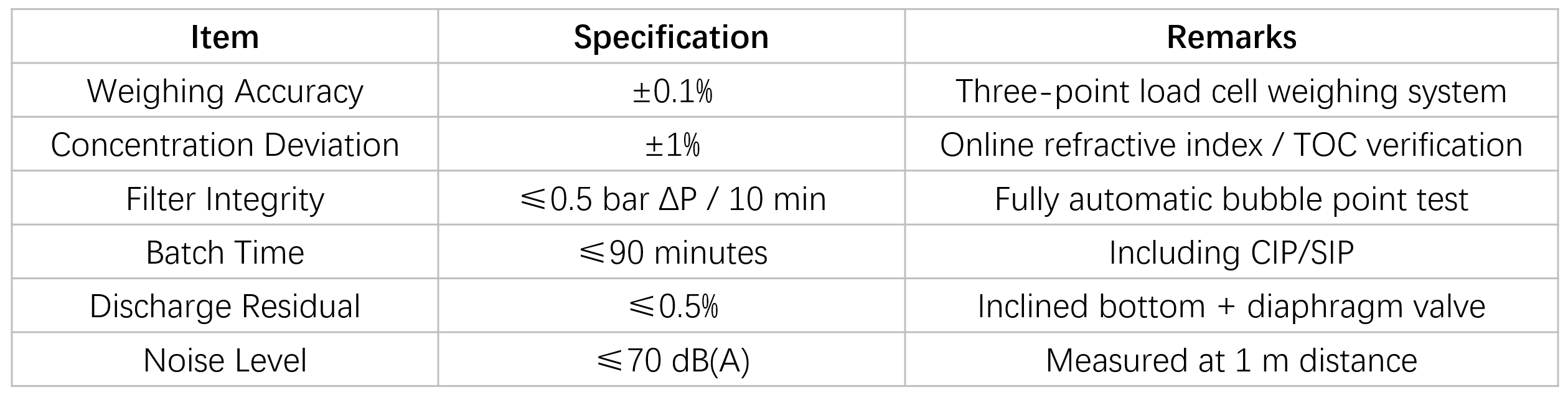
Construction & GMP Design
316L stainless steel three-dimensional frame, electropolished with Ra ≤0.4 μm, sloped surfaces ≥3° for zero dead-leg drainage.
Fully enclosed RABS enclosure with tempered glass viewing windows; all seals made of USP Class VI EPDM / PTFE.
Fully modular piping system with 100% automatic TIG welding and borescope inspection.
Dual-loop jacketed vessel / heat exchanger design, temperature control accuracy ±1 °C.
Optional integration of CIP skid and WFI loop, enabling a closed-loop self-cleaning and self-sterilizing system.
Control System
Siemens S7-1500 PLC + 15" HMI, ≥100 recipes with hierarchical user access management.
21 CFR Part 11 compliant: audit trail, electronic signatures, one-click PDF batch report generation.
Reserved OPC UA and Modbus-TCP interfaces for seamless integration with MES / LIMS and Electronic Batch Records (EBR).
Optional AI-assisted formulation optimization, based on historical concentration–yield big data.
Utility Requirements

GIVE US A MESSAGE



